Radiation Health Risks
Part I – Mechanisms and Biological Effects
Ionizing radiation may well be the most important
single cause of cancer, birth defects and genetic disorders..
The stakes for human health are very, very high in radiation matters.
-John Gofman
Radiation is a broad subject with great depths. It can become technical and nuanced very quickly. We aim to provide a practical understanding of radiation as well as risk reducing measures to be received by a wide audience. Our focus will be to keep the discussion as simple as possible while being relevant to everyone as we should all be concerned with the health risks of radiation. To go just deep enough to explain how radiation can have adverse effects on our health and provide measures that we can take to protect ourselves.
This Article Will Discuss:
- The Mechanisms of Ionizing Radiation (Part I)
- The Biological Effects of Ionizing Radiation (Part I)
- A High-Level View of Radiation Dosage (Part II)
- Man-Made Sources of Ionizing Radiation (Part II)
- The Importance of Iodine Supplementation (Part III)
- Other Ways to Reduce Radiation Health Risks (Part III)
This Article Will Not Discuss:
- Health Statistics
- Radiation Measurements and Metrics (Grays, Sieverts, Decay Rates etc..)
- Non-Ionizing Radiation (generally considered safe or having acceptable health risks)
- Cosmic Radiation
- Historical Developments
Mechanism of Ionizing Radiation
Going Sub-Atomic
Though radiation health risk is no small matter, it has everything to do with one of the smallest components of matter which is the atom. Your body is made up of tissues, which are made up of cells, and your cells contain DNA. DNA, and everything else in physical universe, is made up of atoms. Atoms are made up of protons, neutrons and electrons. Protons and neutrons make up the nucleus of the atom and almost all of the atom’s mass is in the nucleus. Electrons orbit the nucleus. There is a big empty space between the nucleus and the electrons. The size of this space varies by the element but to get a sense of scale here are some analogies.
- If an atom is the size of the earth, the nucleus would be the size of a football field
- If an atom is the size of the New York City, the nucleus would be the size of an orange
- If an atom is the size of a baseball field, the nucleus would be the size of a pea
Protons have a positive electric charge, electrons have a negative electric charge, and neutrons have no electric charge (they are neutral). When the number of protons and electrons are equal, that atom as a whole has a balanced charge and is electrically neutral. If the number of electrons and protons are different, then it has an overall negative or positive charge and is called an ion.
A given element can have different combinations of protons and neutrons. These variations are referred to as isotopes. Isotopes for a given element all have the same number of protons but have a different number of neutrons.
Radioactivity
When the number of protons and neutrons are the same or almost the same the atom tends to be stable and not in a state of radioactive decay. The more the number of protons and neutrons vary the more they tend to be unstable. These are referred to as radioisotopes because they are in a state of radioactive decay. Radioactive decay is the process of losing energy (or emitting radiation) by ejecting particles or energy. This continues until the atom’s nucleus reaches a stable state.
Ionizing Radiation
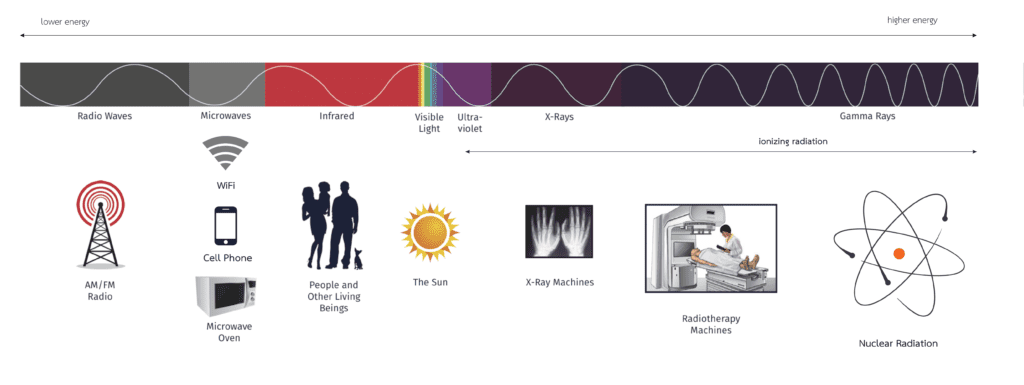
Ionizing radiation is matter and energy released by atoms that travel in the form of particles and energy waves. This type of radiation has enough energy to cause chemical changes in cells and their genetic material DNA. Some cells may die or become abnormal. By damaging the DNA contained in the body’s cells, radiation can cause cancer. Our bodies are very efficient at repairing the cell damage. The success of the repairs depend on the extent of the damage. The extent of the damage depend on the amount and duration of the exposure, as well as the organs exposed. It is also a matter of chance because some components of DNA are more critical than others. Ionization radiation include UV-rays, X-rays and Gamma-rays which are on the upper-end of the electromagnetic spectrum.
3 Types of Radiation
There are 3 types of ionizing radiation: alpha-particles, beta-particles and gamma-rays. Each have a way to create charged atoms.
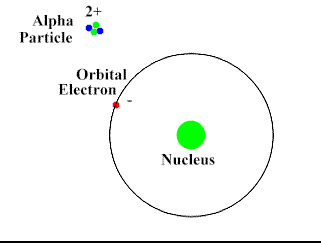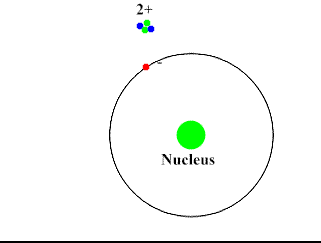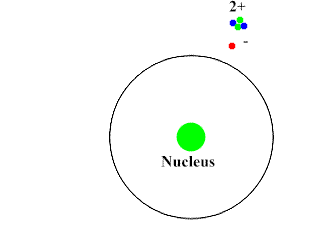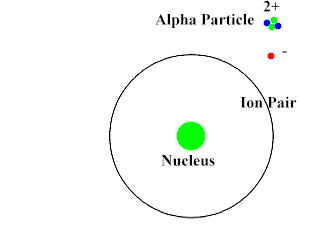
Alpha-Particles are bigger, slower and do not penetrate the skin and most surfaces. They move at about 10% the speed of light. They have a positive charge and attract the negatively charged electrons (opposites attract). Alpha-Particles can draw an electron away from the atom with its attractive force.
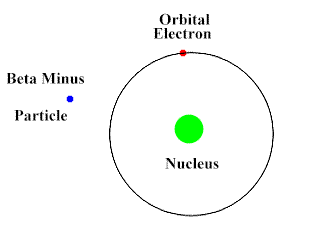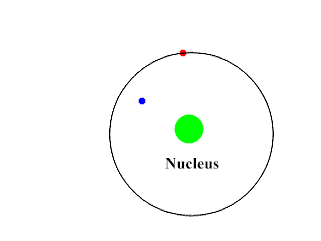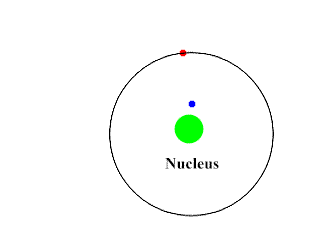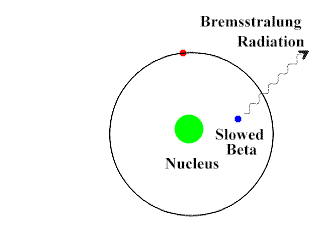
Beta-Particles are smaller, faster and penetrates the skin as well as some other tissues. They move at about 90% the speed of light. They have a negative charge and repel electrons. Beta-Particles can push an electron away from the atom with its repulsive force.
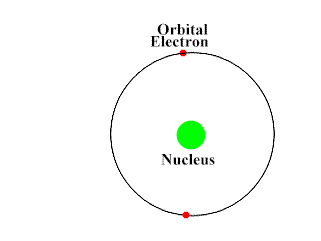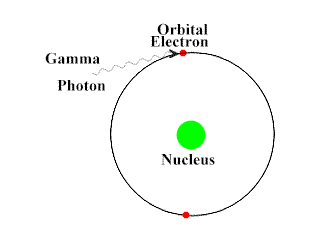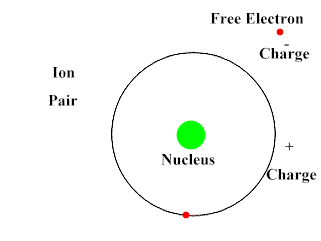
Gamma-Rays are waves of pure energy and have no mass. They penetrate all tissues in the body and many other substances such as wood and some amounts of concrete and steel. Gamma-Rays can hit electrons directly and upon absorbing energy the electron leaves the atom’s orbit.
Upon interacting with an atom, all three types of radiation can dislodge an electron from an atom’s orbit making the atom an ion (or ionizing the atom). For the rest of the article the radiation we are referring to is ionizing radiation which has the most significant health risks.
.
Biological Effects of Ionizing Radiation
Changing the Atom’s Structure
Ionizing radiation are particles and energy waves that have enough energy to change the structure of the atom. It can potentially alter DNA structure either directly or indirectly by first altering other materials in the body. When high energy particles or waves interact with the atom, the atom absorbs the energy and loses an electron. With the number of protons now exceeding the number of electrons the atom becomes positively charged. This represents a change in the atom’s structure and can disrupt the bonding of a group of atoms.
DNA Damage
DNA is a molecule (it is made up of atoms) and carries the genetic instructions used in the growth, development, functioning and reproduction of all known living organisms. You can think of damaged DNA in terms of a computer hard drive with important and sensitive data that becomes corrupted. Having corrupted data can cause poor functionality and stop it from working altogether. Radiation can damage DNA directly as well as indirectly.
Direct DNA Damage
DNA can become corrupted when atoms in the DNA molecule become ionized and the bonding of these atoms are disrupted.
Indirect DNA Damage
Though DNA plays a very important biological role, our bodies consist of very little DNA. Our body is mostly made up of the water molecule H2O. Radiation will often interact with water molecules, causing them to be ionized and creating free-radicals in the process. Free-radicals are atoms or groups of atoms that have an odd number of electrons. Free-radicals are unstable, and in an attempt to become stable, they try to capture the needed electron from other compounds. This leads to a domino-effect of free-radical creation and damage with iterations of chemical damage throughout the body. Free-radicals can damage virtually any molecule in our body including proteins, fats and DNA.
DNA Repair: For Better or Worse
In some cases the cell can recognize the corrupted DNA and carries out the noble act self-destruction. Sometimes the cell attempts to quickly repair the damage. When these repairs fail and the cell does not recognize the failure, it may eventually attempt to divide using the corrupted data leading to genetic diseases, mutations, and/or cancer.
Radiation can have three biological effects each which different outcomes.
- damaged cells repair themselves successfully and return to being normal and healthy
- cells die and are replaced with normal and healthy cells
- cells incorrectly repair themselves and become mutated
Healthy cells dying as a result of radiation may not seem like a good thing. Especially if too many cells die and this hinders or halts the function of the tissue. Impaired functioning of tissues and/or organs can produce acute effects such as skin redness, hair loss, radiation burns, and acute radiation syndrome. The effects are more severe at higher levels of radiation and also with more sensitive tissues. However, dead cells cannot cause cancer.
Cells with damaged DNA that are successfully dividing and creating mutated cells could lead to much worse outcomes in the long run. Rather than causing cell death, the change in the structure of atoms from radioactivity can lead to damaged DNA, cells and tissues. Both cell death and mutations can be immediately observed has radiation sickness and radiation poisoning. Mutations are also observed over a longer period of time in the form of cancer.
Ionization is the process of creating charged atoms. Such changes in the structure of atoms, and consequently how they bond to other atoms, also change the structure of molecules (such as DNA). This can bring about undesirable results in cells and tissues.
In Part II we will discuss the factors that contribute to radiation dosage and the sources of ionizing radiation.
.
.